Institutional Animal Care & Use Committee
Mission
The Sam Houston State University(SHSU) Institutional Animal Care and Use Committee (IACUC) oversees all aspects of SHSU animal care and use programs for teaching and research. The President of SHSU makes all IACUC appointments. The IACUC reviews all animal use protocols, ensures compliance with federal regulations, inspects animal facilities and laboratories, and oversees animal training and educational programs. The IACUC serves as a resource to faculty, investigators, technicians, students, staff, and administrators and provides guidance for conducting all animal use procedures with the highest scientific, humane, and ethical principles.
Regulatory Charges
- Overseeing the use of vertebrate animals for research and teaching
- Establishing policies for ethical animal use and ensuring that such use is compliant with federal, state, and local regulations
- Reviewing all animal use protocols and modifications, requesting approval for the use of vertebrate animals
- While animal welfare is the responsibility of every individual working with animals at SHSU, the IACUC is mandated to investigate and ensure correction of all reported animal welfare concerns and report any animal welfare issues to the Institutional Official and the regulatory agencies as required.
- Overseeing animal husbandry and veterinary care provided to animals on the SHSU campus.
- Overseeing training of faculty, students, and staff on the use of experimental animals.
- Inspecting all areas where animals are housed and used regularly.
- Reviewing the institution’s program for animal care and use at least twice a year (semiannually).
- Performing post-approval monitoring of approved animal use protocols and modifications.
Resources
Welcome to the Cayuse IACUC resources page! To learn more about how to submit your proposals in the new system, there are resources listed below to get you started after you have registered for access to the system. Cayuse IACUC is only supported in the Chrome, Safari, and Firefox browsers.
Please note that effective September 1, 2020, the IACUC office will no longer accept proposals using the old paper application forms. Any faculty or student conducting research involving live vertebrate animals must transition to the new Cayuse IACUC electronic application submission system. To access Cayuse Animal Oversight, click the second button at the top of this page..
To apply for access to Cayuse Animal Oversight please fill out the registration form by clicking the button at the top of this page.
Cayuse IACUC Training Resources
Forms
Animal Care and Use Form X - Exempt Review Application
AALAS Learning Library
The AALAS Learning Library provides training that is essential for faculty, staff, and students working with animal in a research or education setting. SHSU currently only has access to the free courses found here. Please consider incorporating this valuable training into your courses or research programs.
AWIC Alternatives Literature Search
Need assistance conducting an alternatives literature search? The USDA National Agricultural Library’s Animal Welfare Information Center (AWIC) offers free alternatives literature searching support. Fill out their request form and email it along with your animal use protocol (if you are able to) to awic@usda.gov. The request form can be found at the top of AWIC’s Literature Searching webpage under “Request a Literature Search”. You can also use this webpage for step-by-step guidance on conducting your own alternatives search, frequently asked questions, and other resources. If a search is requested from AWIC, results are typically returned within 10 business days.The IACUC will not be meeting during the summer unless absolutely necessary. All Animal Use Protocols (AUPs) will be processed by the Designated Member Review (DMR) procedures. Please allow for up to 2 weeks for a typical AUP DMR review. If you have questions/concerns, please contact Sharla Miles.
Effective immediately, the IACUC has unanimously approved that all current IACUC submissions MUST be received by the ORSP by 5:00 pm on the Monday of the week an IACUC meeting is to occur.
IACUC applications are processed in the order received, and typically take two to three weeks to be processed. The Institutional Animal Care and Use Committee meets in full approximately once a month during the fall and spring semesters.
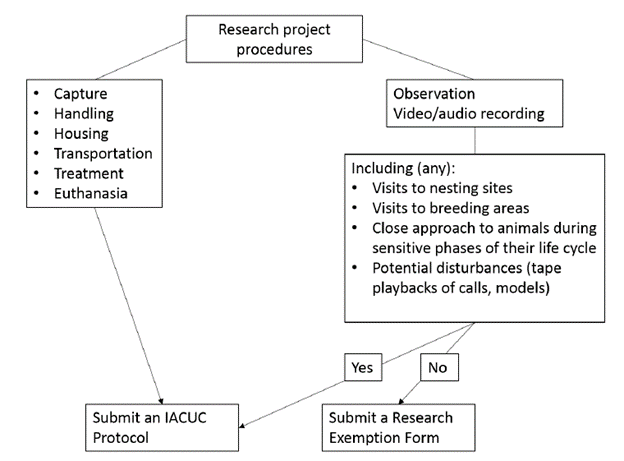
IACUC Exempt Review Flowchart
Official SHSU PHS#: D19-01078
Public Health Service Information
No activity involving animals may be conducted or supported by the PHS until the institution conducting the activity has provided a written Assurance acceptable to the PHS, setting forth compliance with this Policy. Assurances shall be submitted to the Office of Laboratory Animal Welfare (OLAW), Office of the Director, National Institutes of Health. The Assurance shall be signed by the Institutional Official. OLAW will provide the institution with necessary instructions and an example of an acceptable Assurance. All Assurances submitted to the PHS in accordance with this Policy will be evaluated by OLAW to determine the adequacy of the institution's proposed program for the care and use of animals in PHS-conducted or supported activities. On the basis of this evaluation, OLAW may approve or disapprove the Assurance, or negotiate an approvable Assurance with the institution. Approval of an Assurance will be for a specified period of time (no longer than five years) after which time the institution must submit a new Assurance to OLAW. OLAW may limit the period during which any particular approved Assurance shall remain effective or otherwise condition, restrict, or withdraw approval. Without an applicable PHS-approved Assurance no PHS-conducted or supported activity involving animals at the institution will be permitted to continue.
For the purpose of writing grant proposals, the Public Health Service (PHS) assurance number for Sam Houston State University is #D19-01078.
All SHSU personnel (faculty, staff, and students) named in an IACUC protocol who are using live vertebrate animals and need access to SHSU’s vivarium are required to enroll in SHSU’s Occupational Health and Safety Program (OHSP). This entails completing Occupational Health Evaluation and Risk Inventory forms. To enroll in the OHSP, please complete the forms below.
SHSU and Concentra Health Evaluation forms
Personnel must submit the completed Concentra and SHSU health forms via encrypted email to Concentra Medical Center who will work with the designated health care physician for obtaining specific recommendations. The health forms, which will include the physician’s recommendations (if any) will be securely scanned into SHSU’s Concentra portal, which is in keeping with federal, state and local HIPAA regulations.
When the IACUC Administrator receives the physician’s recommendations through the secure portal, this information will be logged into the database, and the recommendations from the health care provider will be mailed to the researcher(s).
Additionally, all personnel must complete a risk assessment questionnaire to be submitted to the OHSP Administrator (Sharla Miles) who will work with EHS for obtaining specific recommendations. All completed risk inventory questionnaires, which will include any EHS recommendations (if any), will be securely stored in the SHSU IACUC office for all personnel working with animals. Before access to the vivarium will be granted, both sets of recommendations must be on file with the OHSP Administrator.
Medical clearance from the OHSP must be completed before any new IACUC protocols will be approved.
If you have any questions regarding the Occupational Health and Safety Program, please contact Sharla Miles by email
Send EmailAnimal Welfare Concerns
Sam Houston State University is committed to the humane and ethical treatment of animals. In the case of emergencies or to report concerns related to the care and use of animals, animal health, or environmental conditions, please contact one of the following numbers below.
WHAT TO REPORT?
- Animals being used for research or instruction without an IACUC approval, or
- An incidence of animal abuse or neglect on Sam Houston State facilities or by Sam Houston State students, faculty, or staff
HOW TO REPORT?
Call one of the following individuals:
- Dr. Chad Hargrave, Associate Provost/Chief Research Officer 936-294-1538
- Dr. Marcy Beverly, IACUC Chair 936-294-1222
IF YOU PREFER TO REMAIN ANONYMOUS
Anyone with knowledge about potential non-compliance with laws, regulations, or policies, or ethical violations is encouraged to report their concerns. Two methods are available. Learn more here.
TO REPORT AN EMERGENCY SITUATION
To report an emergency situation involving an animal on Sam Houston State property:
-
-
- Contact the University Police Department 936-294-1800
-
All reports will be referred to the SHSU IACUC for review, and if warranted, investigated to determine the appropriate corrective actions needed.
Frequently Asked Questions
1. How frequently should the IACUC review research protocols?
The PHS Policy at IV.C.5. states "the IACUC shall conduct continuing review of activities covered by this policy at appropriate intervals as determined by the IACUC but not less than once every three years (3)". The USDA regulations 9 CFR 2.31(a)(5) (4) states "The IACUC shall conduct continuing reviews of activities covered by this subchapter at appropriate intervals as determined by the IACUC, but not less than annually."
2. What steps do I need to take to comply with the continuing review policy?
Submit SHSU IACUC Form F—Annual Review Form to the IACUC office at iacuc@shsu.edu.
3. What is the SHSU policy of IACUC protocol submissions?
Since most IACUC submissions are reviewed via Designated Member Review, there is currently no set submission deadline in place. However, if your procedures fall into the USDA pain/distress category E (e.g., Pain or distress or potential pain or distress that is not relieved with anesthetics, analgesics and/or tranquilizer drugs or other methods for relieving pain or distress), your submission will require Full Committee Review. Hence, those IACUC submissions must be received by the ORSP by 5:00 PM on the Monday of the week an IACUC meeting is scheduled to take place.
4. What is the typical timeline for IACUC review and approval?
Applications are reviewed in the order they are received. The time required for review and approval of an average IACUC application, carefully prepared in accordance with the recommended guidelines, submitted by an applicant who has completed the required Animal Subjects training, is approximately two to three weeks. Full Committee Review (FCR) may take longer depending on when the application is received. Final approval is dependent upon requested modifications, if any, being satisfactorily completed. Many low-risk Animal Use Protocols (AUPs) will be processed by the Designated Member Review (DMR) procedures. Please allow for up to 2 weeks for a typical AUP DMR review and up to 4 weeks for a typical AUP FCR review.
5. Is Post-Approval Monitoring required?
Yes, post-approval monitoring is required per PHS Policy (Section IV.C.(5) of this policy) and the Guide for the Care and Use of Laboratory Animals (Guide pages 33-34).
6. May the IACUC grant conditional or provisional approval?
No. PHS Policy only allows the IACUC to do the following actions pertaining to protocol review determinations: approve, require modifications, or withhold approval. If the IACUC determines that a protocol is approvable, contingent on receipt of a very specific administrative modification or clarification (e.g., a contact telephone number), the Committee may handle the issue as an administrative detail that an individual (e.g., IACUC Chair or Administrator) may verify. Requests for substantive modifications should result in the protocol coming back to the Committee. Applying descriptors, such as conditional, provisional or interim when referring to IACUC approval is not allowed.
1. What records is the IACUC responsible for maintaining?
The IACUC is responsible for maintaining:
- minutes of IACUC meetings;
- records of IACUC attendance, activities, and deliberations;
- documentation of protocols reviewed by the IACUC and proposed significant changes to protocols, and whether approval was given or withheld;
- report of semiannual IACUC evaluations and recommendations to the IO; and
- accrediting body determinations
2. How long are IACUC records to be maintained?
All records are to be kept for a minimum of 3 years, with the exception of records that relate directly to protocols which must be kept for the duration of the activity and for an additional 3 years after completion of the activity.
Records documenting such activities as the provision of adequate veterinary care, training, and occupational safety, are expected to conform with the recommendations of the Guide and with commonly accepted professional standards.
An adverse event is any occurrence, usually involving pain, distress or death of an animal, which was not described in the approved IACUC Protocol or its subsequent modifications that has a negative impact on animal welfare. An IACUC protocol deviation is any departure from the methods approved in the IACUC protocol. Note that the IRB further distinguishes between protocol violations and deviations; however, the IACUC does not.
2. Why should AEs be reported?
Reporting AEs assists principal investigators, animal care staff and the attending veterinarian to find the cause and to prevent recurrence. Reporting also helps the IACUC meet its federal requirement to monitor animal activities.
3. Who should report AEs?
PIs and animal facility directors should report to the IACUC as soon as they become aware of an event that may impact animal welfare. An email or phone call is recommended for a preliminary report as soon as possible after the event. The final report should be made on the official form within 7 calendar days and after consultation with veterinary staff.
4. What qualifies as an AE?
When in doubt, call the animal facility director or the IACUC to discuss the event. Unexpected events or problems are considered AEs if they affect greater numbers of animals than anticipated, have a negative impact on other animals or activities, or reflect a situation that could become more severe in the future. A report is not required if the event and its management are described in the approved IACUC protocol.
5. What types of events must be reported?
The following events must be reported:
- Morbidity or mortality resulting from complications not described in the IACUC protocol.
- Greater number of mortalities, more severe responses, or when animals appear to be in more pain or distress than expected/described in the IACUC protocol. For example, a report would be required, if 10 % of animals die following surgery when a 5% fatality rate was indicated in the approved protocol.
- Allergic reaction to a treatment; inadequate anesthesia; development of an unexpected infection following surgery or treatment.
- Facility or equipment failure that has a negative impact on animal welfare. Loss of electrical power impacting HVAC function or water supply; restraint equipment malfunction; biohazard containment failure. Facility design, husbandry or postoperative care that has a negative impact on animal welfare
- Entrapment; overexposure to heat source(s); inadequate analgesia or antibiotic use.
- Off-protocol activity, or Protocol Deviation, i.e., any intentional or unintentional use of animals that was not described in the approved IACUC protocol.
Injury or illness unrelated to approved procedures and being treated by the attending veterinarian or designee. Events that are described in the approved protocol that occur at rates that are equal or below the rates indicated in the approved protocol.
7. What information needs to be reported?
Information required includes the project title and IACUC protocol number; the principal investigator’s name; the date, time, location and nature of the event; measures taken at the time to minimize impact on animal welfare; the actual or potential impact of the event on animal welfare and study outcomes; and immediate and long-term steps being taken or considered to prevent recurrence of the event. The name and signature of the person reporting the adverse event also are required. The aforementioned information should be requested in the Form J—Adverse Event and Protocol Deviation Form, which you must use to submit this report. Click here to find this form.
8. How should reporting of a typical AE proceed?
See below:
- In an emergency, contact a staff veterinarian immediately.
- Consult with the animal facility manager to provide any necessary changes in animal care.
- Communicate as soon as possible with IACUC by phone or e-mail to inform them of the situation and receive further instruction as appropriate.
- Work with veterinary staff to complete this report including a Corrective Action Plan, as needed, and submit it by email to IACUC within 7 calendar days of the event.
- The attending veterinarian, or designee, will review the report and seek further clarification or sign off that it has been fully resolved.
- The report will be included in the agenda for the next IACUC meeting and the IACUC chair will seek further clarification or sign off that the event has been documented by the IACUC, thereby closing the issue.
9. What will the IACUC do with the report?
The purpose of AE reporting is to document at the level of IACUC, in accordance with its federally mandated role of oversight, that adverse events have been fully addressed by the research team and veterinary staff as they occur. Timely reporting demonstrates the commitment of the research team to provide the highest quality animal care by engaging all available resources. A subcommittee of the IACUC will review these reports in detail prior to consideration at a convened IACUC meeting. Most of these reports will be informational to the full IACUC; others may require further action. Amendments to approved protocols may be necessary to modify procedures based on knowledge gained from adverse events. In some cases, it will be advisable for principal investigators to voluntarily halt certain animal procedures until an event is fully addressed. If adverse events are not appropriately addressed in a timely manner, the IACUC has the responsibility and authority to protect animal subjects with actions up to and including the suspension of approved protocols. Failure to report adverse events is noncompliance with IACUC policy. As official IACUC documents, these reports are subject to Texas open records requests at any time.